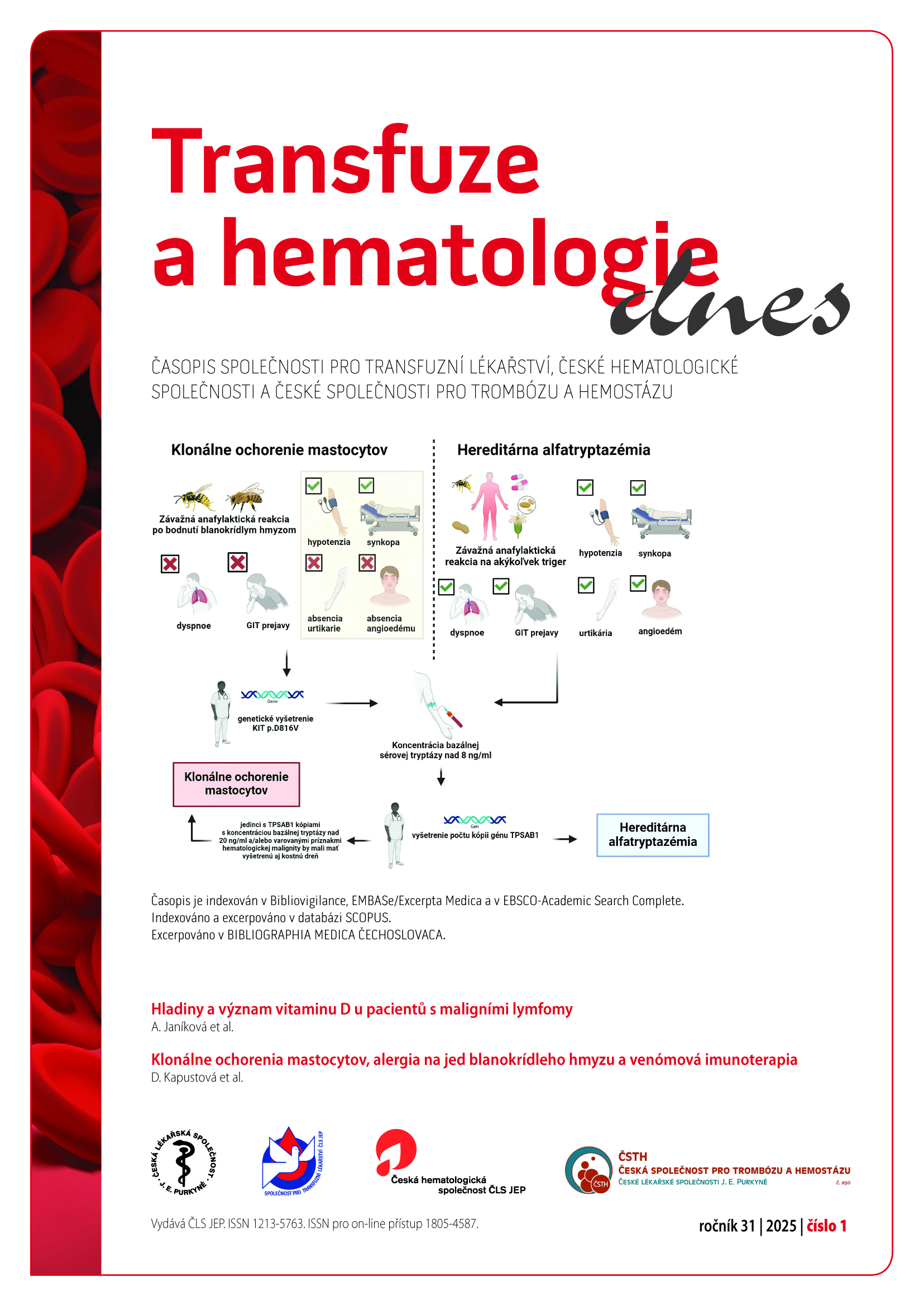
Clonal mast cell disorders, hymenoptera venom allergy and venom immunotherapy
Keywords:
clonal mast cell proliferation disorders, hymenoptera venom, venom immunotherapy, systemic mastocytosis, hereditary alpha-tryptasemia, serum basal tryptaseAbstract
Introduction: Clonal mast cell disease and/or increased basal serum tryptase concentration are among the risk factors for a more severe life-threatening course of an anaphylactic reaction after a Hymenoptera insect sting. Because of the riskiness of these patients, the implementation of a single causal therapy – venom immunotherapy (VIT) should be recommended for each such patient.
Material and methods: We created a prospective study consisting of 93 patients (58 men, 35 women) who met the indication criteria for VIT treatment. We included patients in the study gradually and data were collected from 2015 to 2023. We defined the basic characteristics of the group, we focused on the identification of patients with an elevated concentration of serum basal tryptase (˃ 8 ng/ml) and systemic mastocytosis, the specifics of the course of their systemic allergic reaction after a Hymenoptera insect sting, treatment tolerance, and occurrence of adverse effects.
Results: From a total of 93 enrolled patients treated with VIT, we recorded persistently elevated concentrations of serum basal tryptase (sBT) ˃ 8 ng/ml in 15 patients (16,7 %). We diagnosed systemic mastocytosis in one patient. In a patient with elevated sBT values, we most often observed loss of consciousness and hypotension during a systemic allergic reaction after a Hymenoptera sting (p = ns). From the point of view of treatment tolerance, we observed a roughly comparable incidence of adverse effects in terms of local reactions. A total of 53,3 % of patients treated with VIT with elevated concentration of sBT overcame natural re-exposure to Hymenoptera insects, while not a single patient developed a systemic allergic reaction requiring the administration of an adrenaline autoinjector.
Conclusion: Hymenoptera venom allergy is a life-threatening condition, and its occurrence is much more frequent, and the course is more severe in patients with elevated basal serum tryptase concentrations and/or clonal mast cell disease. In each patient with a confirmed clonal mast cell disease, it is necessary to search for the occurrence of an allergic reaction to Hymenoptera insect venom in the patient’s history and to consider the indication for venom immunotherapy as the only causal treatment.
Key words: Clonal mast cell proliferation disorders, Hymenoptera venom, Venom immunotherapy, Systemic mastocytosis, Hereditary alpha-tryptasemia, Serum basal tryptase